MPE launched the Myeloma and AL amyloidosis Community Taskforce to address a key concern of patient advocates – better representation of the patient voice throughout the drug research, development and approval processes.
MPE Myeloma and AL amyloidosis Community Taskforce
What is the Myeloma and AL amyloidosis Community Taskforce?
We have seen all too often that patients are not engaged or consulted in drug development, research, and regulatory processes – despite patients being the ones whose lives are most affected by the arrival of new treatments and access to those treatments. MPE works with the pharmaceutical industry and other important stakeholders, to address this issue and advocate for greater involvement of patients. The Myeloma and AL amyloidosis Community Taskforce is one important means of ensuring that patients can provide input in a meaningful way and help shape how therapies are developed and delivered.
As a standing committee of patient experts and advocates, the Taskforce is prepared to respond in a timely manner to ensure that recommendations are incorporated based on stakeholder timelines. The Taskforce is comprised of patients and advocates from across Europe, representing the diversity of the patient community.
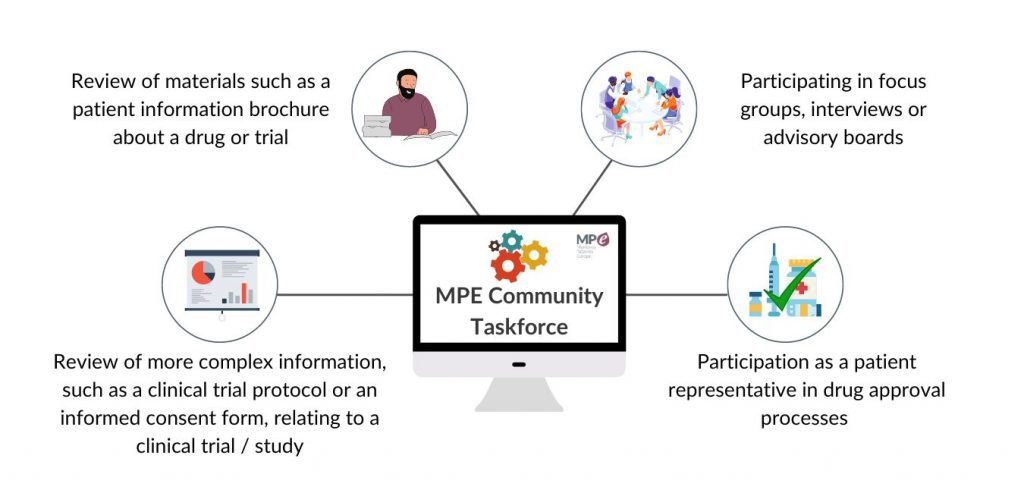
What does the Myeloma and AL amyloidosis Community Taskforce do?
There are many activities that sit within the Taskforce. These include:
All members of the Community Taskforce receive extensive training from MPE staff to learn how to review materials and provide constructive feedback, as well as individual support when needed. Taking part in Taskforce activities is entirely voluntary, and members decide which activities are right for them. For some activities, Taskforce members will receive compensation for their time and efforts, and members are informed of this per activity.
How do I get involved?
Subscribe to
our newsletter!