Table of Contents
Why is this topic important?
There have been many recent advances in the management of myeloma. As new treatments have emerged, this has led to improved outcomes for myeloma patients. However, despite this promising progress, infection remains a leading cause of death for myeloma patients. This is recognised in the myeloma community, and medical organisations have produced guidelines based on evidence to support doctors and nurses, patients and patient advocates to help manage the impact of infection on myeloma patients. Myeloma itself leads to an increased risk of infection, but many myeloma treatments can also contribute to further increasing this risk. Some of the most recently developed and effective medications (directed at immune cells called T-cells) are associated with even higher infection risk. These include CAR T-cell therapies and bispecific antibody medications. It is therefore important for myeloma patients and carers to be informed of these increased risks to empower them to make informed decisions about their care and be aware of the safety profile of these medications.
What is an infection?
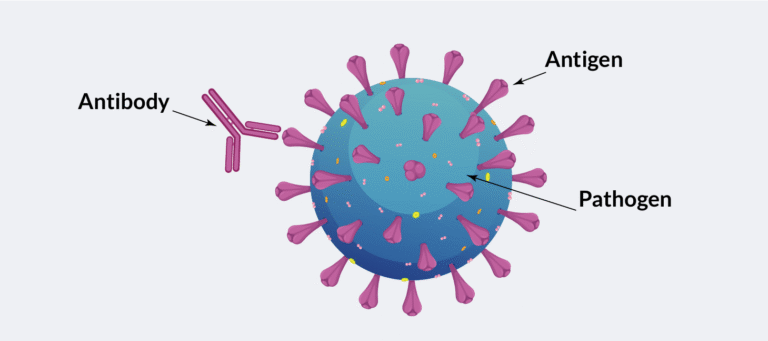
Causes of infection, otherwise known as pathogens, include viruses, bacteria and fungi. Infection happens when pathogens enter the body and begin to multiply, making you unwell. Infections can cause a variety of symptoms depending on where they are located. General symptoms of infection include fever, chills, pain, fatigue and feeling generally unwell. Infections are usually treated with medications called antimicrobial medications. Antibiotics, which fight bacteria, are a common example. Serious infections may require treatment in hospital.
How is myeloma related to the immune system?
The immune system protects the body from causes of infection. There are many parts of the immune system in different parts of the body. Some parts form the initial resistance against a pathogen. This is called the innate immune system. The innate immune system includes barriers to the outside world such as skin. It also includes some types of white blood cells such as neutrophils. These react to pathogens non-specifically to fight infection. Neutrophils and other innate immune cells are located throughout the body and blood, ready to react.
The body can also mount a more specific immune response to pathogens. This is called the adaptive immune system. The body does this using other types of white blood cells such as lymphocytes and plasma cells. Plasma cells are found in bone marrow (inside large bones) and certain other tissues. These cells form an immune response to pathogens by producing antibodies. These antibodies are proteins made to stick to the specific pathogen, marking them for destruction. Myeloma develops when normal plasma cells become cancerous and divide in an uncontrolled manner. These cells disrupt the normal function of the immune system and increase risk of infection.
Myeloma has a direct impact on the function of the immune system. Having myeloma means that the normal response to infections is disrupted. Myeloma patients are more likely to get infections when compared to people of the same age without myeloma. The immune system of a person with myeloma is not as effective at fighting infection as those without myeloma. Infection remains a leading cause of death in myeloma patients.
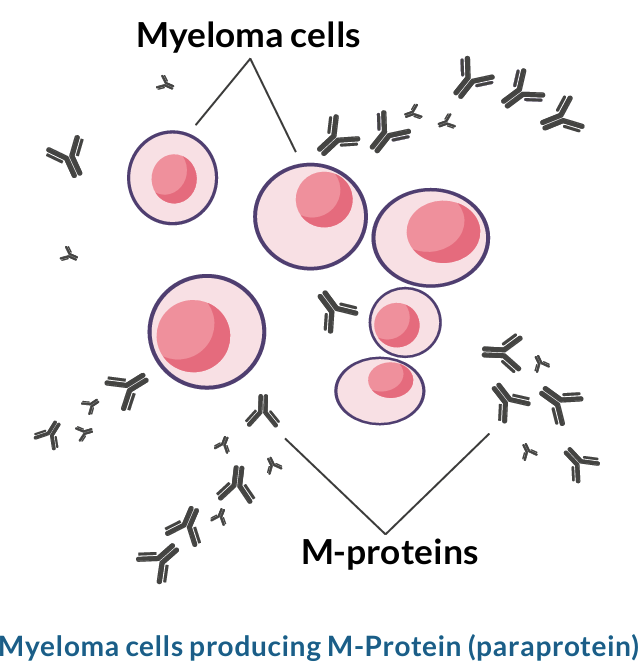
In myeloma, there are often fewer immune cells, and these immune cells are less effective. The myeloma cells (cancerous plasma cells) are primarily located inside the bone marrow. The cells do not leave enough space for a normal number of plasma cells or other immune cells to be made in the bone marrow, because the myeloma cells grow out of control. Additionally, it has been observed that immune cells function less effectively due to myeloma. Myeloma cells try to produce an antibody, but the antibody produced does not help to fight infection. The Myeloma cells M-proteins overproduced, non-helpful antibody is known as M-protein or paraprotein.
Myeloma also causes other issues in the body that lead to increased risk of infection. Kidney problems can occur due to raised calcium levels (through bone being damaged by the myeloma cells). Also, the kidney’s delicate filtering tubes can become clogged with the paraprotein that myeloma cells produce. When the kidneys are damaged, the impact of infection tends to be higher.
What are other risk factors for infection in patients with myeloma?
- Age. Although it can occur in younger people, patients who develop myeloma tend to be older. Older age is linked with reduced immune function even in those without myeloma. Older people are also more likely to have other problems that affect the immune system, such as lung disease, kidney disease and frailty. These factors can increase the risk of significant infections.
- Prior exposure to viruses. Those who have been exposed to certain viruses in the past may be at risk of a reactivation infection. A patient who develops myeloma may have been exposed to pathogens in the past that lay dormant while the immune system functions well. When myeloma occurs, the immune system becomes compromised, and these viruses can reactivate. This does not happen for all previous viral infections, only specific examples. For example, conditions such as shingles can occur when myeloma develops. Shingles is caused by the chicken-pox virus (the virus is called varicella zoster) and leads to a painful rash.
- Pre-existing health conditions. Patients with pre-existing conditions that lower immunity or result in organ damage are more at risk of developing infections and dying from them. For example, patients with diabetes and autoimmune disorders (such as rheumatoid arthritis), patients with HIV, patients who are malnourished (meaning the body is deficient in essential nutrients) and patients with other cancers, have weakened immune systems due to these conditions, meaning they may pick up infections more easily. Patients with heart disease, kidney disease, or other organ damage have fewer reserves to fight infection when it occurs, meaning the infection is more likely to worsen. This is because infection causes organ damage and strain.
How do myeloma medications increase risk of infection?
Many medications for treating myeloma increase the risk of infection. The increased risk occurs for different reasons with each medication and to varying degrees. The choice and combination of medications is dependent on many factors, such as test results, patient fitness and preference, medication availability, prior medication exposure and refractoriness to certain medications.
More about the choice of medications for myeloma patients can be found in the MPE Myeloma Patient Guide. The most common medications and associated infection risks are detailed per medication class in the following section. Medications that use the patient’s own immune system (CAR-T and bispecific antibody medications) increase the risk of infection more than other medications.
CAR T-cell therapy
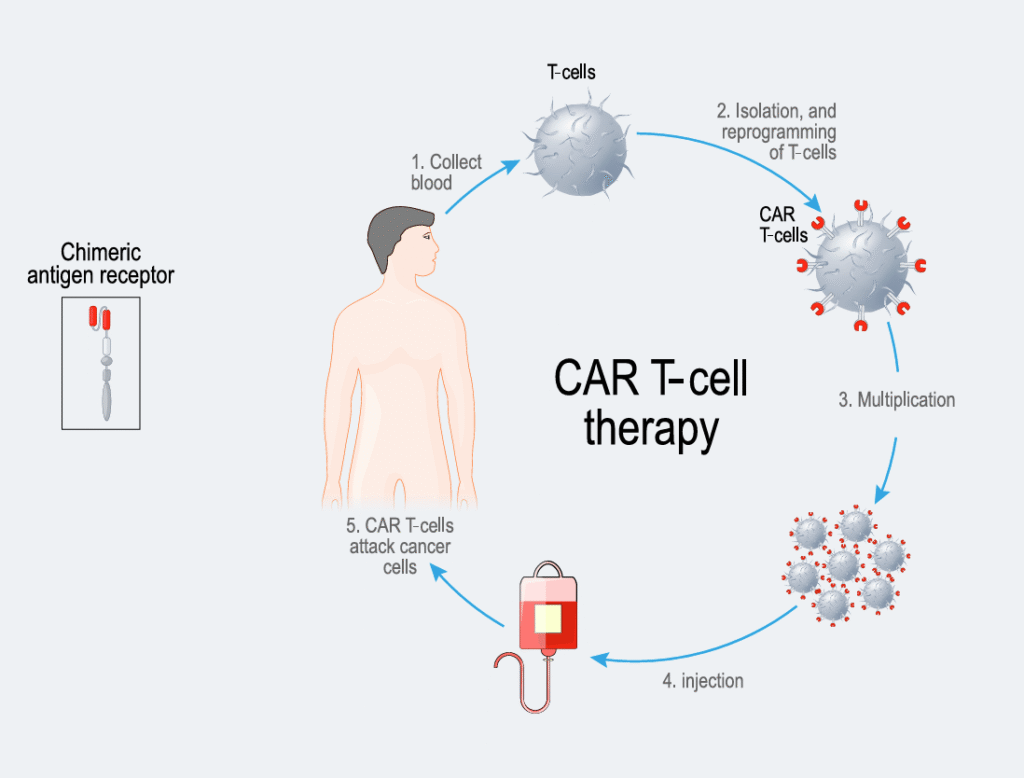
CAR T-cell therapy is a type of immunotherapy. Immunotherapy uses the patient’s immune system to target the myeloma cells. A patient’s own T-cells (a type of white blood cell) are modified to include a protein called a “chimeric antigen receptor” (CAR), which helps the immune system find and destroy myeloma cells. In the current approved CAR T-cell therapies for myeloma, the CAR protein binds to the B cell maturation antigen (BCMA) protein on the surface of the myeloma cell, which then allows the immune system to kill the myeloma cell. CAR T-cell therapies are widely approved for relapsed/refractory myeloma patients. Relapsed/refractory myeloma is the term used for when myeloma returns after treatment (relapsed myeloma), or when myeloma does not respond to treatment (refractory myeloma). The two CAR T-cell therapies approved (i.e., have marketing authorisation) in Europe for relapsed/refractory myeloma are ciltacabtagene autoleucel, approved for second line and beyond myeloma treatment, and idecabtagene vicleucel, approved for third line and beyond myeloma treatment. Infection is considered a leading preventable cause of death during CAR T-cell therapy. Clinical trials have shown that for both the approved CAR T-cell therapies in myeloma, more than half of patients in the clinical trials developed at least one infection and around 20% had an infection classed in the category of severe to life-threatening.
Infection risk is high before, during and after the CAR T-cells are infused into the body.
- Before CAR T-cell infusion, medications are given to deliberately lower the number of other immune cells (called lymphocytes) to improve the chances of the CAR T-cells working against the myeloma. Lymphocytes fight off infections, particularly viral infections.
- Soon after CAR T-cell infusion (up to day 30), patients are likely to have low immune cells including neutrophils (neutropenia) which are part of the first line of defence in fighting infection, particularly against bacteria. Many patients also develop cytokine release syndrome (CRS) in this phase. CRS is where the body reacts and makes lots of inflammatory proteins (cytokines), and causes symptoms like fever, aches and dizziness, and affects the whole body. CRS can prolong the time where the immune cells are low and sometimes requires further immune suppression to treat, further increasing risk of infection.
- After day 30, the issue of low immune cells may persist, and the lymphocyte white blood cells often do not work properly for a few months, depending on the patient. One reason for this is because the CAR T-cells can result in the destruction of normal immune cells, as well as myeloma cells. This can result in immunoglobulins (pathogen targeting proteins) not being produced as normal in a condition called hypogammaglobulinemia (which means low immunoglobulin levels). This tends to increase the risk of infections and can persist long-term (for over a year) after CAR T-cell therapy.
The role of CAR T-cell therapy in treating myeloma is likely to become more substantial in the future. Therefore, understanding how to effectively prevent and manage infections during CAR T-cell therapy is increasingly important.
Bispecific antibody medications
Bispecific antibody medications are a type of immunotherapy that work by binding to both the myeloma cell and an immune cell called a T-cell. This allows the immune cell to kill the myeloma cell. Examples of bispecific antibodies include teclistamab, talquetamab, and elranatamab. Bispecific antibody medications have an increasingly important role in the treatment of relapsed/refractory myeloma
and clinical trials are exploring their use in earlier lines of treatment.
Bispecific antibodies increase risk of infection through a similar mechanism to CAR T-cells; by lowering the levels of normal immune cells through the same mechanism they use to kill myeloma cells. Patients often experience low immunoglobulin levels (hypogammaglobulinemia), low white blood cell counts and reduced function of immune cells.
Clinical trials have shown that patients taking bispecific antibody medications have high rates of all severity levels of infection, and moderate rates of severe infection. An analysis of 11 clinical trials studying bispecific antibody medications in myeloma patients demonstrated that over an average period of six months, half of patients experienced infection and 25% experienced severe to life-threatening infection.
Bispecific antibody medications targeting the BCMA protein on myeloma cells, for example, teclistamab and elranatamab, have been shown to cause higher rates of infection than bispecific antibody medications with different targets such as talquetamab, which targets a protein called GPRC5D. This is thought to be because the BCMA protein is important in making immunoglobulins and for normal immune cell function.
Furthermore, when bispecific antibody medications are combined with other myeloma medications, such as daratumumab (a monoclonal antibody medication) and lenalidomide (an immunomodulatory medication) risk of infection is higher than when administered alone.
Monoclonal antibody medications
Monoclonal antibody medications, for example, daratumumab and isatuximab, are designed to recognise and bind to the CD38 protein, which is found on the surface of myeloma cells. By attaching to CD38, daratumumab kills myeloma cells through direct anti-tumour activity and also activates the immune system to kill the myeloma cells. It is thought that anti-CD38 monoclonal antibody medications increase the risk of infection by lowering several different types of white blood cell count. One review of 11 clinical trials reported that patients taking anti-CD38 monoclonal antibody medications combined with other standard treatments are 1.27 times more likely to acquire infection, compared with patients taking the same standard treatments alone. However, no increased risk of death from infection was found.
Proteasome inhibitor medications
Proteasome inhibitor medications cause proteins to build up to toxic levels within myeloma cells, which causes them to die. Bortezomib and carfilzomib are examples of proteasome inhibitor medications. Increased infection risk is caused by the medications lowering the levels of white blood cells that would usually fight infection.
Immunomodulatory medications
Immunomodulatory medications (IMiDs) include thalidomide, lenalidomide, and pomalidomide. They have multiple mechanisms of action that work on the immune system, including blocking the development of myeloma cells and directing the immune system to kill myeloma cells. Increased infection risk is caused by the medications lowering the levels of white blood cells that would usually fight infection.
Iberdomide and mezigdomide are from a newer medication class called CELMoDs (cereblon E3 ligase modulatory drugs) that are in the research phase of development and build on the established platform of immunomodulatory medications. In a phase I/II study of mezigdomide plus dexamethasone for patients with relapsed/refractory myeloma, 65% of patients experienced some level of infection (mild to severe), likely owing to the high rates of neutropenia (77% of patients).
Selective inhibitor of nuclear export (SINE) medications
Selinexor, an oral medication, is currently the only myeloma medication in the selective inhibitor of nuclear export (SINE) medication class. It is given with dexamethasone after fourth line treatment to patients with myeloma progression, who are refractory to certain other medications. It is also given with dexamethasone and bortezomib after first line myeloma treatment. Selinexor binds to the exportin 1 (XPO1) protein, which helps move proteins out of the cell nucleus. Selinexor blocks the transport of several proteins involved in cancer cell growth from the cell nucleus to the rest of the cell. This leads to the death of the myeloma cells. Clinical trials have shown that selinexor can cause severe neutropenia in around 20% of patients, which would increase the risk of infection in these patients.
Chemotherapy
Chemotherapy works by interrupting the process of cancer cell division. For example, cyclophosphamide (a common medication in myeloma treatment combinations), causes damage to the cancer cell DNA, which causes the cell to die. However, all rapidly dividing cells are affected by the medication, leading to low immune cells and increased risk of infection.
Melphalan is another form of chemotherapy, which is most commonly given at a high dose as part of a stem cell transplant. Due to the side effect of reducing immune cells, the infection risk is high, (amongst other adverse effects) and therefore this treatment is usually only administered to younger/fitter patients. In a phase III clinical trial of 389 patients, 19% of patients who had undergone a stem cell transplant involving high-dose melphalan treatment experienced severe/life threatening infections.
Recent developments include the approval of melphalan flufenamide in combination with dexamethasone to treat relapsed/refractory myeloma patients who have had three or more lines of therapy. It is a first-in-class peptide drug conjugate – a treatment that combines a chemotherapy drug (melphalan) and a peptide (a small protein). Adding a peptide to melphalan helps the melphalan more rapidly and efficiently find and enter myeloma cells. A phase II clinical trial found that 11% of patients with relapsed/ refractory myeloma developed severe/life-threatening infections and 79% of patients developed neutropenia.
Steroids
Steroids, or corticosteroids, are an effective part of many myeloma treatment combinations. They reduce the body’s inflammatory response by mimicking a natural hormone called cortisol. Dexamethasone is a common example. The inflammatory response is part of the body’s natural response to infection, so the infection risk is increased, especially for higher doses. There are also many other side effects, such as mood disturbance. One research study of 445 patients with newly diagnosed myeloma found that a lower dose of dexamethasone (combined with lenalidomide, an immunomodulatory drug) was associated with better short-term survival and lower rates of infection than when higher doses of dexamethasone were combined with the same dose of lenalidomide.
How will my infection risk change during my myeloma treatments?
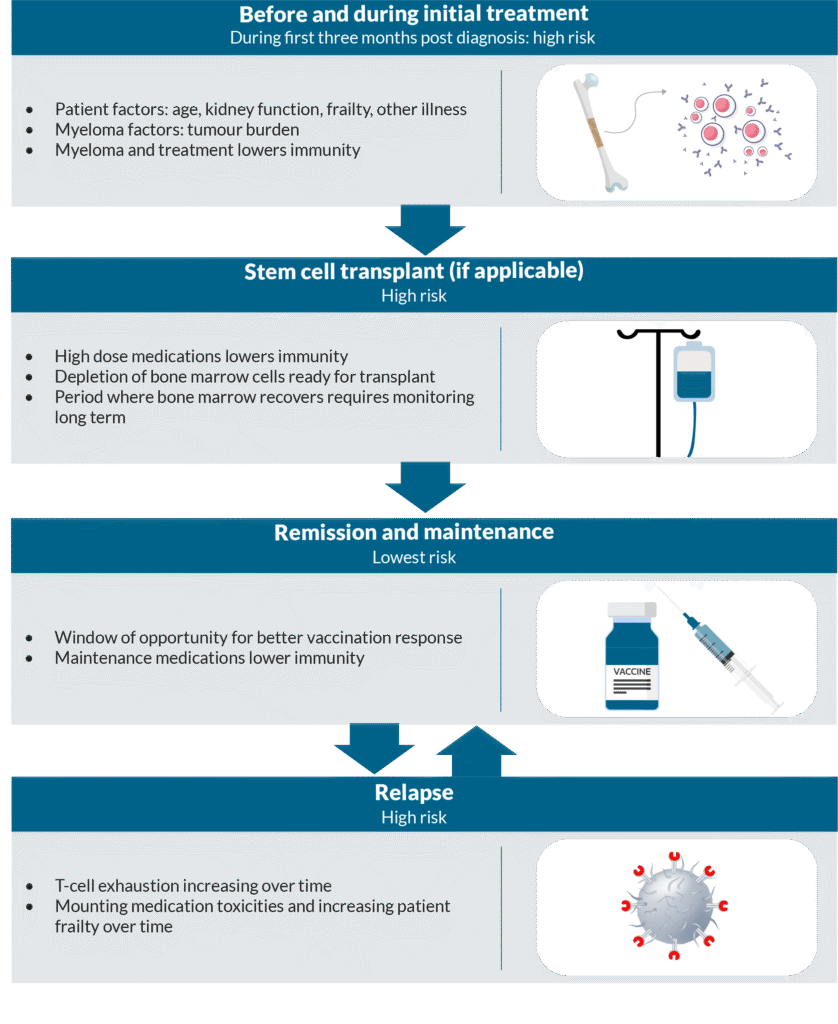
The risk of infection fluctuates depending on your treatments and on whether your myeloma is in remission, meaning there is improvement or temporary disappearance of your myeloma after treatment.
Before and during initial treatment
On average, the infection risk is highest during the first three months after diagnosis of myeloma. One large Swedish study reported that infection contributed to 32% of deaths two months after diagnosis. Furthermore, 2.6% of all patients died from infection related illness within two months of diagnosis. During initial treatments, immunity is lowered by the various medications and myeloma cells are still present, further reducing immunity. Interventions to reduce infection risk, such as preventative antibiotics, may be started and patients should be made aware of the signs of infections to be vigilant of. Individuals have different susceptibility to infection depending on factors such as how much myeloma is present (tumour burden), overall health, kidney function, age, frailty, choice of treatment and treatment intensity.
At stem cell transplant
Patients who have stem cell transplants have usually had 4-6 months of treatment with a standard of care regimen prior to this. Before a transplant, high dose chemotherapy (melphalan) is given to deplete cells in the bone marrow ready for the stem cells to repopulate. This causes the risk of infection to be high, particularly with bacterial infections. The risk remains high until the stem cells have started working to produce enough immune cells again. Patients may be given specific advice and medications to reduce infection risk during this time and afterwards for up to 3-12 months.
Maintenance therapy and remission
A patient’s immunity level usually improves significantly during myeloma remission, because normal healthy immune cells are restored to a degree. The risk of infection is considered at its lowest during this time. However, the adaptive immune system that produces antibodies “forgets” how to react to common pathogens due to the disease and its treatment, especially after stem cell transplant. It is important for patients to be offered vaccinations, especially during remission phases, as they work best during this time. Immunity to common pathogens that may have been damaged during stem cell transplant can be built up by undertaking re-vaccination (vaccines that may have been given previously). Patients who take maintenance therapy such as lenalidomide will be at risk of having low immune cells, which increases the risk of infection.
At first and subsequent relapses
During periods of relapse patients are at high risk for acquiring serious infection. These periods are associated with a similar infection risk to when a patient is first diagnosed. Fungal infections may develop more commonly than in other periods of having myeloma, as well as risk of bacterial and viral infections. Mounting toxicities due to medication and increasing patient frailty are thought to contribute. T-cells are a key part of adaptive immunity to pathogens, and therefore prevention of infections in the body. Having myeloma and myeloma medications causes these cells to become tired over time, because they are being stimulated more frequently than if a patient did not have myeloma. Therefore, they are less effective at fighting infections over time. This is called T-cell exhaustion and adds to risk of infection during treatment of relapsed/refractory myeloma. T-cell exhaustion also affects how well the body can kill myeloma cells. This is an active topic of research, as it is linked with reduced effectiveness of T- cell engaging medications such as bispecific antibody medications. Research is needed to understand whether giving the T-cells a break, by changing dosing schedules, can help to reduce the risk of infection.
Summary
- Myeloma and its treatments reduce immunity and increase risk of infection.
- Infection is a leading cause of death for myeloma patients at any stage.
- Patients with myeloma are usually at their highest risk of infection during the first three months after diagnosis and during periods of relapse.
- Infection risk is usually lowest during periods of remission.
- T-cell directed therapies such as CAR T-cell therapies and bispecific antibody medications are effective treatments that have a higher infection risk than other medications.