What is linvoseltamab (Lynozyfic®)?
Linvoseltamab is a myeloma treatment that was granted conditional marketing authorisation from the European Commission in April 2025. This means it is safe and effective for use in myeloma patients.
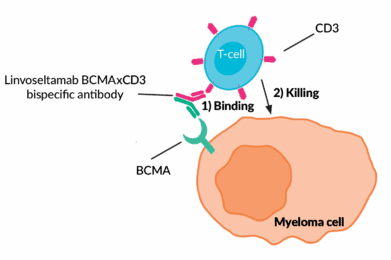
Linvoseltamab is a laboratory-made B-cell maturation antigen (BCMA)-CD3 bispecific antibody.
Conditional marketing authorisation is granted to new medicines based on less comprehensive clinical trial data than normally required if the medicine addresses an unmet medical need and if its benefits outweigh the risks. Researchers must provide more comprehensive clinical data in the future to maintain approval status.
Linvoseltamab has been conditionally approved as treatment on its own (i.e., a monotherapy) for myeloma patients with relapsed and/or refractory myeloma, who have received at leas t t h re e p r ior t h e r apie s , in cl u di n g a n immunomodulator y agent (IMiD) (such as lenalidomide or pomalidomide), a proteasome inhibitor (such as bortezomib or carfilzomib) and an anti-CD38 antibody (such as daratumumab or isatuximab). Patients must also have evidence of progressed disease since their last therapy.
How does linvoseltamab work?
Linvoseltamab is a bispecific antibody, meaning it targets two proteins at the same time. It is designed to find a protein on the surface of myeloma cells called B-cell maturation antigen (BCMA). BCMA is highly expressed on the surface of myeloma cells, making it an effective target. At the same time, it also binds to the CD3 protein on the surface of immune cells, known as T-cells.
Through this bispecific interaction, linvoseltamab brings the T-cell and myeloma cell in close contact with one another, allowing the T-cell to initiate an immune response and kill the myeloma cell. Linvoseltamab works in a similar way to other BCMA targeting bispecific antibodies such as teclistamab (Tecvayli®) and elranatamab (Elrexfio®).
What are the benefits of linvoseltamab?
Linvoseltamab’s efficacy was investigated in a phase II study called LINKER MM1. The clinical trial enrolled 117 patients with relapsed and/or refractory myeloma who had received at least three prior lines of treatment or were triple-class refractory; they were treated with linvoseltamab 200 mg until their myeloma returned (i.e., disease progression). It was not compared with any other treatment.
71% of patients responded to linvoseltamab treatment, with 50% of patients having a complete response (i.e., no detectable monoclonal protein in the body) or better. Responses were maintained for 29 months in more than 50% of cases. Patients with high-risk myeloma, over 75 years old or refractory (i.e., no longer responding) to up to five main myeloma treatments also responded well to linvoseltamab. The clinical trial is still ongoing to assess the time before the disease progresses and how long patients survive after taking this treatment. Other trials will evaluate linvoseltamab’s efficacy compared to standard treatments and in combination with other myeloma treatments.
What are the side-effects of linvoseltamab?
The most common serious side effects of linvoseltamab, which may affect more than 10% of patients are:
■ CRS (cytokine release syndrome) which may include fever, feeling dizzy or light-headed, chills, difficulty breathing, or a fast heartbeat
CRS occurs when the immune system is activated by bispecific antibodies like linvoseltamab or by CAR T-cell therapy. CRS is given a grade to describe the severity of the adverse events with grade 1 being a mild reaction, treated with supportive care only, grade 4 being life-threatening complications and grade 5 being fatal. In the LINKER-MM1 study, 46% of patients experienced CRS, mostly at low grades (one patient had a grade 3 CRS, and no patient had higher grade CRS).
■ Infections and blood disorders which can increase infection risk
- pneumonia (lung infection), with symptoms such as cough, fever, difficulty breathing, or chest pain
- COVID-19 infection, with symptoms such as fever, chills, cough, difficulty breathing, sore throat, fatigue, or a new loss of taste or smell
- urinary tract infection, with symptoms such as fever, chills, pain or a burning feeling when passing urine, an increased urge to pass urine, or back pain
- sepsis (severe infection throughout the body, <10% of patients)
- CMV infection (can cause serious blood and tissue infections, <10% of patients)
- hypogammaglobulinaemia (low levels of antibodies called ‘immunoglobulins’ in the blood)
- neutropenia (low levels of a type of white blood cell)
- febrile neutropenia (neutropenia with a fever, <10% of patients)
In the LINKER-MM1 study, infections occurred in 73% of patients, with 34% of patients having a grade 3-4 infection (severe to life-threatening). 11 patients died due to infection. Infection frequency and severity declined over time.
■ infusion-related reactions, which may include fever, feeling dizzy or light headed, chills, difficulty breathing, or a fast heartbeat (<10% of patients)
■ ICANS (immune effector cell-associated neurotoxicity), a serious immune reaction which includes indicators such as trouble speaking, writing, or understanding things, feeling confused or being less alert or aware, feeling disoriented, seizures (7% of patients in the LINKER-MM1 study)
Other very common side effects, which may affect more than 20% of patients include (sorted by frequency):
■ anaemia (low levels of red blood cells)
■ cough
■ diarrhoea
■ fatigue (feeling exhausted or persistently tired)
■ muscle and joint pain
■ hypokalaemia (low potassium levels)
■ nausea (feeling sick)
■ headache
■ back pain
■ dyspnoea (being short of breath)
How and when is linvoseltamab given?
After step-up dosing, linvoseltamab is administered as an intravenous infusion (IV – in the vein). It is given once a week for the first 14 weeks, and then every two weeks. Depending on how the patient responds to treatment, it can be given every four weeks starting from week 24. Treatment is then continued until disease progression or until side effects are not tolerated by the patient anymore. Any active infection will need to be treated before treatment initiation. The duration of the first infusion is over four hours and will be decreased to one hour and then to 30 minutes, if well tolerated.
The treatment will be started and supervised by a trained healthcare professional, and administered in a facility with immediate access to emergency equipment and medical support to appropriately manage side effects if they occur. Patients receiving linvoseltamab will be given an alert card with important information on CRS and ICANS risks, and instructions on when to seek emergency help or advice.
To reduce side effects, the treatment dose is progressively increased for the first three weeks to reach the full target dose on week three. This is called a step-up dosing schedule. Patients are closely monitored within close proximity of a qualified treatment centre for safety for 24 hours after the first two step up doses and after the second step-up dose in case some adverse events were experienced with the first one. Hospitalisation is not required but might be
necessary in certain circumstances. Medicines are also given before treatment to prevent side effects until patients have two full doses without these side effects occurring. Those pretreatments include dexamethasone (to avoid inflammation), antihistamine (to avoid allergic reactions) and paracetamol (to avoid fever). Given the risk of infection, prophylactic treatments are recommended to prevent infections from occurring, depending on local institutional guidelines. Recommended prophylactic strategies include: intravenous (or subcutaneous) immunoglobulins, antivirals (i.e. medicines that help the body fight against certain viruses that can cause disease) and antimicrobials (i.e. medicines that kill or stop the growth of microorganisms (bacteria or fungi) that can cause disease. Linvoseltamab treatment can be delayed or stopped in case severe side effects occur.
References
For a full list of references used to inform this factsheet, please email info@mpeurope.org