CAR-T is a form of immunotherapy currently being investigated for the treatment of myeloma. Immunotherapy drugs use the body’s immune cells to fight cancer.
What is CAR-T therapy?
Chimeric antigen receptor T cell (CAR-T) treatment uses a type of white blood cell known as T cells. T cells play vital roles in the body’s immune response to infection or cancer. Cancer cells commonly evade the immune system, making them difficult to find and destroy. During CAR-T therapy, T cells are modified to find and destroy cancerous myeloma cells. T cells are manufactured and equipped with a protein molecule called a “chimeric antigen receptor” (also known as CAR) to enable T cells to find and eliminate myeloma cells. CAR is a protein that resides on the surface of T cells and can instruct T cells to find, bind to and destroy cancerous myeloma cells. Equipping the T cells with the CAR protein is a complicated process involving the genetic modification of T cells in a laboratory. Specific methods are used to introduce the CAR DNA into the T cell. These methods include using viruses or other vectors, which carry the CAR DNA into the T cell.
CAR-T is currently under investigation for various stages of myeloma, but at this time, most patients treated with CAR-T are considered relapsed/refractory, meaning they have exhausted most of their available treatment options, and when a patient’s disease has either stopped responding or is not responding to other available treatments.
What are the steps for CAR-T treatment?
CAR-T therapy is a multi-step process. The usual procedure is as follows:
Evaluation: You will be evaluated to determine whether CAR-T therapy is a suitable option. Several factors, including your age, previous lines of therapy and general fitness, will be considered.
Cell collection: In preparation for treatment with CAR-T, white blood cells will be collected via leukapheresis, which requires a needle to be placed in your arm.
Cell processing: White blood cells, including the T cells, are sent to a laboratory. In the laboratory, the cells are separated to identify the appropriate T cells used to make the CAR-T treatment. Next, the T cells are equipped with the CAR gene.
Cell expansion: The modified T cells are then grown in the laboratory. This process may take two weeks or more. During CAR-T manufacturing, you will continue
treatment with your regular regimen.
Conditioning therapy: Before you receive the CAR-T cells, you will receive chemotherapy (also known as lymphodepleting therapy), which is required to help your body accept the CAR-T cells.
Reinfusion of T cells: After processing, CAR-T cells are reinfused back into your body to multiply, identify the myeloma cells and eliminate them.
Recovery and monitoring: Following CAR-T therapy, you will be closely monitored in the hospital for around two weeks for any side effects and then in the outpatient clinic for approximately three months.
The timeline for the above steps may vary depending on the drug. However, manufacturing CAR-T products takes anywhere between 2-6 weeks. After treatment, a follow-up monitoring phase of 1-3 months is common, but long-term follow-up for several years is required, given that CAR-T is developed using genetic engineering.
What is the difference between an autologous stem cell transplant and CAR-T?
Autologous stem cell transplants (ASCT) and CAR-T therapy are two different treatments for multiple myeloma that share some common features. For example, both treatments involve the reinfusion of cells collected from your body and both require conditioning chemotherapy (i.e. lymphodepletion therapy) to prepare your body for treatment. Despite some similarities, ASCT and CAR-T are unique treatments that differ from each other in significant ways. ASCT is a well-known form of myeloma treatment where stem cells are used to restore the bone marrow after high-dose chemotherapy. CAR-T cell therapy is a new and emerging immunotherapy (the type of treatment that uses your immune system to fight your myeloma). ASCT uses stem cells, and CAR-T uses a type of immune cell known as T cells that are modified to find and destroy cancerous myeloma cells. The mechanism of action of ASCT and CAR-T are also different. ASCT works to replenish healthy stem cells, and where the chemotherapy eliminates your myeloma. CAR-T, by comparison, attempts to remove cancerous myeloma cells using your body’s immune system.
What research has been done to investigate CAR-T?
Research on CAR-T for myeloma is in the early stages compared to other hematologic diseases. The most widely researched myeloma cell target is the B cell maturation antigen (BCMA). BCMA is a protein universally present on the surface of myeloma cells and, therefore, a promising target for CAR-T to bind to and destroy cancerous myeloma cells. The first BCMA-targeted CAR-T cell therapy was developed in 2013 and demonstrated a promising overall response rate (ORR) of 81% ¹. The most significant recent breakthrough of CAR-T for the treatment of myeloma was the US Food and Drug Administration (FDA) approval of idecabtagene vicleucel (also known as Abecma©, ide-cel, or bb2121) in March 2021 and subsequent approval by the EMA in August 2021. Abecma© was studied in 140 patients, 30% of whom had a complete response (low or no presence of abnormal plasma cells on evaluation), and 67% had at least a partial response². Detailed information on Abecma©, including how the medication works, risks/benefits and the approval process, is available here on the EMA website. Additional patient-friendly information on Abecma© can also be found here.
Another noteworthy CAR-T therapy is ciltacabtagene autoleucel (also known as Carvykti©, cilta-cel, JNJ-4528). The FDA approved ciltacabtagene autoleucel in February 2022 for the treatment of patients with relapsed or refractory myeloma after four or more prior lines of therapy, including a proteasome inhibitor (like bortezomib), an immunomodulatory agent (like lenalidomide) and an anti-CD38 monoclonal antibody (like daratumumab or isatuximab). See more information about this approval here. This CAR-T product targets two parts of the BCMA protein on myeloma cells with goals to improve the efficacy of the drug. Cilta-cel demonstrated a 97% ORR in the CARTITUDE-1 clinical trial³. Information on CARTITUDE-1 is available here.
For further details on BCMA targeting CAR-T products, you can follow this link to a comprehensive table summarising BCMA CAR-T clinical trials as of 20204. Aside from BCMA, other promising target proteins on myeloma cells include SLAMF7, CD38, GPRC5D and CD19. For example, MPE is a consortium member of the CARAMBA clinical trial investigating SLAMF7 CAR-T therapy, a Horizon 2020/EU-funded project taking place across six European countries. You can read more about CARAMBA here and in MPE’s pipeline publication. Click here5 for more information on ongoing clinical trials investigating targets outside of BCMA.
How long does CAR-T therapy last?
The progression-free (PFS) time to next treatment and overall survival (OS) vary depending on the specific CAR-T product. Disease remission following CAR-T therapy is, in most cases, no longer than 18 months6. However, this can vary from patient to patient, and with the CAR-T product given that significant improvements are likely to occur with further CAR-T research and advancements.
Who is eligible for CAR-T? Are there any contraindications or age/health restrictions?
The criteria for receiving CAR-T therapy may vary from product to product; however, patients generally must be over 18 years of age, and their overall health status is carefully considered. Most patients considered eligible for CAR-T treatment have a high level of fitness. For example, patients with active viral or bacterial infections, complex medical issues (such as heart, liver, or kidney disease), or autoimmune diseases; or patients with other cancers, as well as pregnant or breastfeeding patients cannot receive CAR-T. In addition, to receive CAR-T most patients must be considered relapsed/refractory and should have received a certain number of previous lines of therapy (including proteasome inhibitors and immune modulators) and their myeloma should have progressed after or during the last treatment. The number of previous lines varies from one study to another and depending on the product. The current label for Abecma© is after three lines of therapy in Europe and four lines in the US, and is being explored as first line of therapy in clinical trials. For more information on inclusion/exclusion criteria for entry into a CAR-T clinical trial, please refer to MPE’s publication on The Reality of CAR-T Therapy: Am I Eligible?
Should caregivers be present during CAR-T?
Generally, caregiver support is necessary during CAR-T therapy, given the complex need for monitoring and follow-up throughout the treatment process and afterwards. It is important to note that patients may not be considered eligible for CAR-T clinical trials or treatment if they do not have the required social support at home. Carers should be aware of the possibility and potential seriousness of the side effects of CAR-T therapy and when they need to seek help. Carers should also be mindful of the potential burden of extended hospital stays, frequent office visits and home monitoring. Carers and patients may have to travel far and potentially miss work to support their loved ones. They should also be aware that their loved ones may be admitted to the intensive care unit as some patients may experience severe side effects.
Are there any dietary restrictions? What are the nutritional requirements?
It is important to know that cancer and treatments can cause loss of appetite, weight loss, diarrhoea, nausea and vomiting, among other symptoms. Given some of the side effects of treatment, proper nutrition before, during and after CAR-T treatment is crucial. Good nutrition with a well-balanced diet may help with strength, energy and overall health. Specific dietary interventions and nutritional support related to CAR-T therapy have not been extensively researched. In other cancer therapies, maintaining nutritional status has been associated with improved patient outcomes7. Therefore, nutrition support and other dietary interventions will likely impact patients receiving CAR-T. The healthcare team administering CAR-T can provide a dietary plan and notify you about any restrictions. Dietary restrictions relate to neutropenia (low levels of neutrophils, a type of white blood cell), which is a side effect of many myeloma treatments, not only CAR-T therapy. In this case, the immune system becomes too weak to fight properly against infections and bacteria, and avoiding contaminants is vital.
Foods to avoid generally comprise raw meat, fish, seafood or eggs; unpasteurised dairy products; unwashed vegetables and fruits; food that has been touched, or can be touched by other people (buffets, restaurants); fresh vegetables and fruits (cooking them is preferable) and undercooked and old food (leftovers >48h). Overall, a well-balanced and healthy diet is recommended for all myeloma patients to support a patient’s health.
Do all patients manage to collect the necessary white blood cells?
During the initial evaluation for consideration of CAR-T cell treatment, T cell and often other blood and myeloma cell counts are drawn. Research has shown that, generally, even from patients who have reduced white blood cell counts due to chemotherapy, doctors often manage to collect a sufficient amount of T cells for CAR-T manufacturing8. Yet, there is still a chance and risk that there may not be sufficient enough T cells to be manufactured. If this was the case, you would be unable to receive CAR-T treatment or may need to undergo subsequent T cell collection, likely delaying the time when you would be able to receive CAR-T, or risking your eligibility for CAR-T.
How do patients tolerate CAR-T? What are the potential side effects?
There are many side effects associated with CAR-T therapy, and each patient may respond differently. Cytokine release syndrome (CRS) is a common side effect of CAR-T therapy. CRS is a systemic inflammatory condition that appears as a flu-like illness and includes the following – but not limited – symptoms: fever (38°C or higher); chills/shivering, fatigue; nausea or diarrhoea; headache; dizziness/light-headedness; shortness of breath; low blood pressure; high or irregular heart rate. CRS is commonly treated with a drug called tocilizumab or, on rare occasions, with steroids. Symptoms may differ for each patient, but they can be fatal, if severe. CRS generally occurs on the same day, or within a week, of CAR-T cell therapy administration, and this is why patients will be monitored closely in the hospital for symptoms during this time, and why outpatient administration is not recommended. Patients stay usually for 7-14 days at the hospital.
Neurotoxicity is another possible, but rare, side effect that may appear with BCMA-targeted CAR-T cell therapy. Symptoms include fatigue; headaches; altered mental state; confusion; agitation; difficulty speaking; slurred speech; loss of balance; tremors and seizures in rare but severe cases. While CRS and neurotoxicity are some of CAR-T’s most common or severe side effects, other side effects are often seen due to conditioning treatment. The CAR-T process, specifically conditioning treatment, involves eliminating cells from your bone marrow – and therefore immune system – such as white blood cells. Indeed, CAR-T treatment is associated with bone marrow suppression in nearly all patients, with prolonged suppression of healthy plasma cells.
This leads to a weakened immune system, with an increased risk of infections. CAR-T can also change the levels of blood minerals (e.g. low potassium, sodium, or phosphate) and lower red blood cell counts and platelets, leading to an increased risk of bleeding, bruising and infections, and causing additional fatigue. Studies have found that up to one-third of patients may experience a bacterial infection in the first 30 days after treatment with CAR-T, and viral infections are also common9. Additionally, patients may also experience infusion-related reactions, including allergies. Due to the potential for severe side effects, some CAR-T cells may be equipped with a safety switch. In patients who experience life-threatening symptoms during or after treatment, EGFR antibodies can be given to the patient and will activate the safety/off switch when possible. For example, the CAR-T cells utilised in the CARAMBA CAR-T clinical trial are unique, because they incorporate an on/off safety switch to mitigate side effects when considered very severe. It is important to quickly report any side effects to the health care team so the symptoms can be appropriately managed.
What should I expect during the recovery phase after CAR-T Therapy?
Due to the potential severity of side effects, you will require hospitalisation for around two weeks or more to receive treatment while remaining under close observation. Also, hospital stays may be prolonged in some cases and may require intensive care unit admission. Due to the need for close monitoring, you and your carers should be aware that frequent hospital and office visits – potentially daily visits – will be required after discharge from the hospital. Patients who live further away from the treatment facility may be required to stay in a hotel or close to the hospital for several weeks for close monitoring. Carers are often required to do frequent checks in the home setting, administer medications and keep a diary of patient symptoms.
What medications are taken during and after CAR-T?
In preparation for treatment with CAR-T, the conditioning or lymphodepletion phase requires patients to receive low-dose chemotherapy to reduce their number of white blood cells. Lymphodepletion is necessary for the patient’s body to accept CAR-T cells. Commonly used lymphodepleting drugs include fludarabine and cyclophosphamide. Following CAR-T infusion, in cases where a patient is showing symptoms of CRS, they will receive steroids, or a drug called tocilizumab, to lessen symptoms. Other medications used to treat the side effects of CAR-T are steroids or an antibody to interleukin-1. Granulocyte colony-stimulating factors (G-CSF) can be given to stimulate bone marrow cell production to increase white blood cell counts, antibiotics to decrease the risk of infection and intravenous fluids for hydration.
Is CAR-T combined with traditional multiple myeloma treatment?
CAR-T is usually administered as a single treatment after lymphodepletion and is not often combined with other myeloma drugs. There are studies investigating the use of CAR-T in the earlier and later stages of myeloma, with or without maintenance treatment, or a stem cell transplant. For example, the CARTITUDE 5 study investigates CAR-T administration in newly-diagnosed patients after induction (or initial) treatment with bortezomib, lenalidomide and dexamethasone, followed by the CAR-T treatment cilta-cel in comparison to the same treatment without cilta-cel10. Several similar studies are also ongoing, such as the KaRMMA 4 study, which can be found here.
What are the consequences of the COVID19 pandemic on CAR-T therapy?
Several factors affected the delivery of CAR-T cell therapies during the pandemic:disruption of healthcare and overwhelmed health workers; issues in the supply chain of commercial CAR-T cells; closing of borders, which created shipping issues; difficult access to ICU and shortage of tocilizumab, which is used in CRS management, and pauses in clinical trial enrolment during lockdowns. 29% of European CAR-T centres delayed therapy in 2020, with 55% of patients delayed for one month, 26% for 1-6 months and 16% who did not receive therapy at all. In 68% of cases, the delay required an additional therapy prior to CAR-T therapy. It seems that the virus cannot be transmitted through the infused product, however, myeloma patients who contracted COVID19 after CAR-T cell therapy had a poor outcome (with a 15 times higher mortality rate). Longer isolation, as well as protection using all available measures (prevention, social distancing, masks, vaccination of healthcare professionals and family members, virtual outpatient visits and priority to products that can be given in an outpatient setting), are recommended. Post-CAR-T vaccination is recommended after at least six months, post infusion. Further work is required to determine the predictors of vaccine response, efficacy and safety in CAR-T recipients.
What is the cost of CAR-T?
The cost of CAR-T treatment in Europe cannot yet be determined, as the first CAR-T product (idecabtagene autoleucel, Abecma©) has just been approved and varies based on each country. However, treatment and health system costs are expected to be high for CAR-T in addition to the cost of hospitalisation and frequent follow-ups. Also, patients and their loved ones should be mindful of ‘ancillary costs’ that may arise, including travel costs, hospital stay costs, nursing care and follow-up visits. Please see this article, which discusses cost concerns with CAR-T treatment in the US.
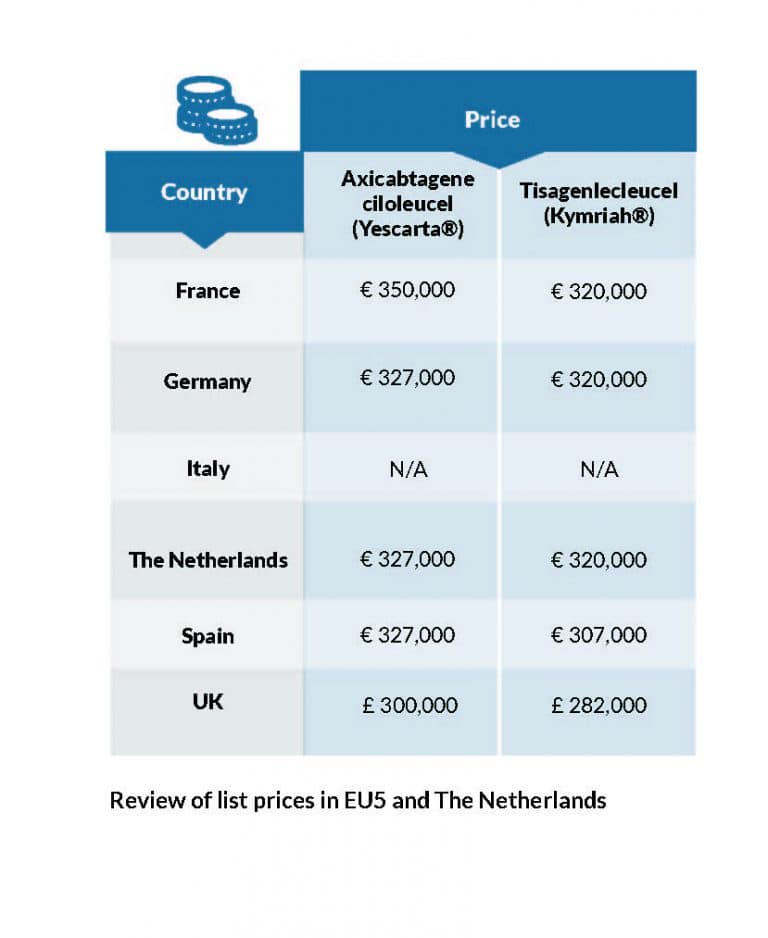
For example, other CAR-T products, Yescarta and Kymriah, were approved several years ago to treat lymphoma (another type of cancer). Kymriah is also indicated for the treatment of acute lymphoblastic leukaemia. Both CAR-T treatments are expensive and their costs are detailed in Figure 1 below:
Figure 1. Review of list prices in EU5 and The Netherlands (table adapted from a presentation given by Dr. Uyl de Groot from the Erasmus University, Rotterdam, atan ESH roundtable, “How I Manage CAR-T Therapies and Bispecific Antibodies for My Patients?” in September 2021.”
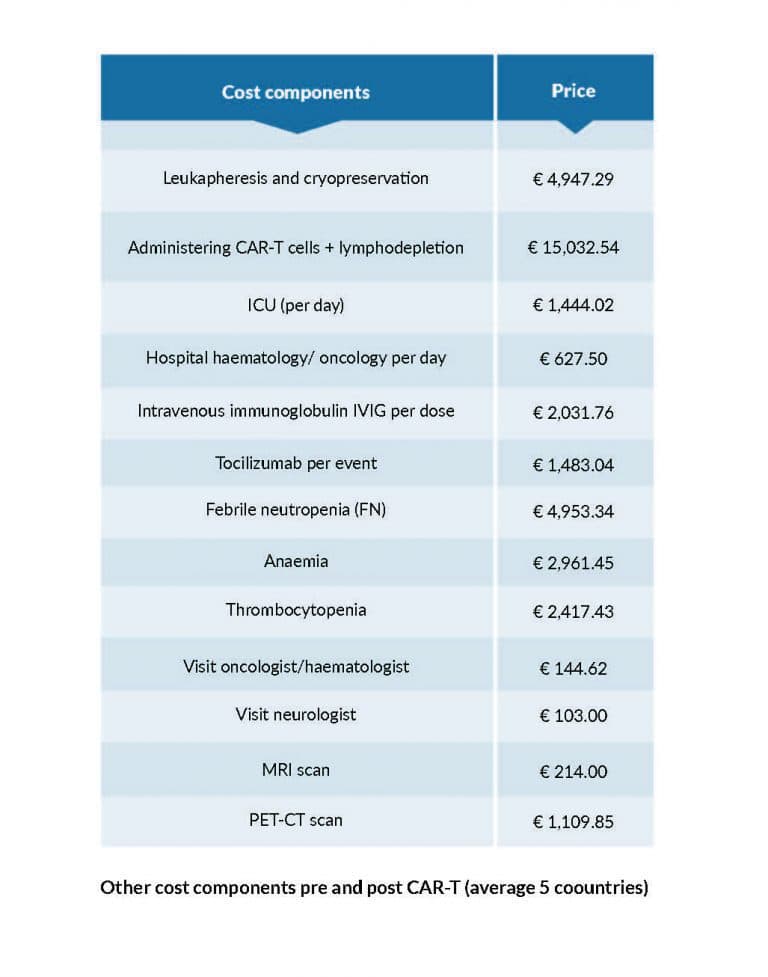
In addition, the costs of hospitalization are an important consideration, and the national health system may cover these costs. The treatment cost components of Yescarta and Kymriah CAR-T products for the treatment of lymphoma patients is detailed in Figure 2 below
Figure 2. Other cost components pre and post CAR-T (average 5 countries) (table adapted from a presentation given by Dr. Uyl de Groot from the Erasmus University, Rotterdam, at an ESH roundtable “How I Manage CAR-T Therapies and Bispecific Antibodies for My Patients?” in September 2021.”
Do patients pay for CAR-T, or is it covered by insurance?
Considering that the first CAR-T product for myeloma (ide-cel, Abecma©) has just gained marketing authorisation (i.e., a license) for use in Europe, individual national reimbursement for this product is unclear. Furthermore, reimbursement schemes for travel costs, hospital stays, and followup costs will most likely vary from country to county. To date, the primary way of accessing CAR-T for treating myeloma is through a clinical trial, in which all the direct (and potentially some ancillary) costs related to the CAR-T treatment would usually be covered by the clinical trial sponsor and the national healthcare system. Marketing authorisation by the European Commission is the first step for a medicine to be made available to patients in Europe, as it determines whether it is safe and effective for use. Once marketing authorisation is granted, national healthcare systems then decide on the value the medicine has to patients in their respective countries and whether to provide reimbursement and therefore, availability in a specific country. European health systems operate very differently and have different models for assessing, approving, and funding medicines. Reimbursement and access to CAR-T depend on the patient’s country, how the health system operates, and the country’s specific reimbursement decision.
MPE will continue monitoring and updating our Myeloma Access Atlas to reflect CAR-T access in Europe. Please email access@mpeurope.org or visit the Myeloma Access Atlas for further information.
References
- Ali SA, Shi V, Maric I, et al. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood. 2016;128(13):1688-1700. doi:10.1182/blood-2016-04-711903
- Abecma. European Medicines Agency. Accessed March 7, 2022. https://www.ema.europa.eu/en/medicines/human/EPAR/abecma
- Martin T. Updated Results from CARTITUDE-1: Phase 1b/2Study of Ciltacabtagene Autoleucel, a B-Cell Maturation Antigen–Directed Chimeric Antigen Receptor T Cell Therapy, in Patients With Relapsed/ Refractory Multiple Myeloma. ASH 2021. Accessed March 17, 2022. https://ash.confex.com/ash/2021/webprogram/Paper146060.html
- Table 2 Anti-BCMA CAR-T-cell clinical trials in multiple myeloma. Teoh, P.J., Chng, W.J. CAR T-cell therapy in multiple myeloma: more room for improvement. Blood Cancer J. 11, 84 (2021). https://doi.org/10.1038/s41408-021-00469-5
- Table 3 Non-anti-BCMA CAR T-cell clinical trials in multiple myeloma (true to the time of writing). Teoh, P.J., Chng, W.J. CAR T-cell therapy in multiple myeloma: more room for improvement. Blood Cancer J. 11, 84 (2021). https://doi.org/10.1038/s41408-021-00469-5
- Mikkilineni L, Kochenderfer JN. CAR-T cell therapies for patients with multiple myeloma. Nat Rev Clin Oncol. 2021;18(2):71-84. doi:10.1038/s41571-020-0427-6
- Cucchiaro B, Weekes CE. Systematic review of nutrition support interventions in adult haematology and oncology patients receiving CAR-T cell therapy. Clin Nutr ESPEN. 2021;46:60-65. doi:10.1016/j.
clnesp.2021.10.015 - Korell F, Laier S, Sauer S, et al. Current Challenges in Providing Good Leukapheresis Products for Manufacturing of CAR-T Cells for Patients with Relapsed/Refractory NHL or ALL. Cells. 2020;9(5):E1225. doi:10.3390/cells9051225
- Stewart AG, Henden AS. Infectious complications of CAR-T-cell therapy: a clinical update. Ther Adv Infect Dis. 2021;8:20499361211036772. doi:10.1177/20499361211036773