What is cytogenetic testing in myeloma?
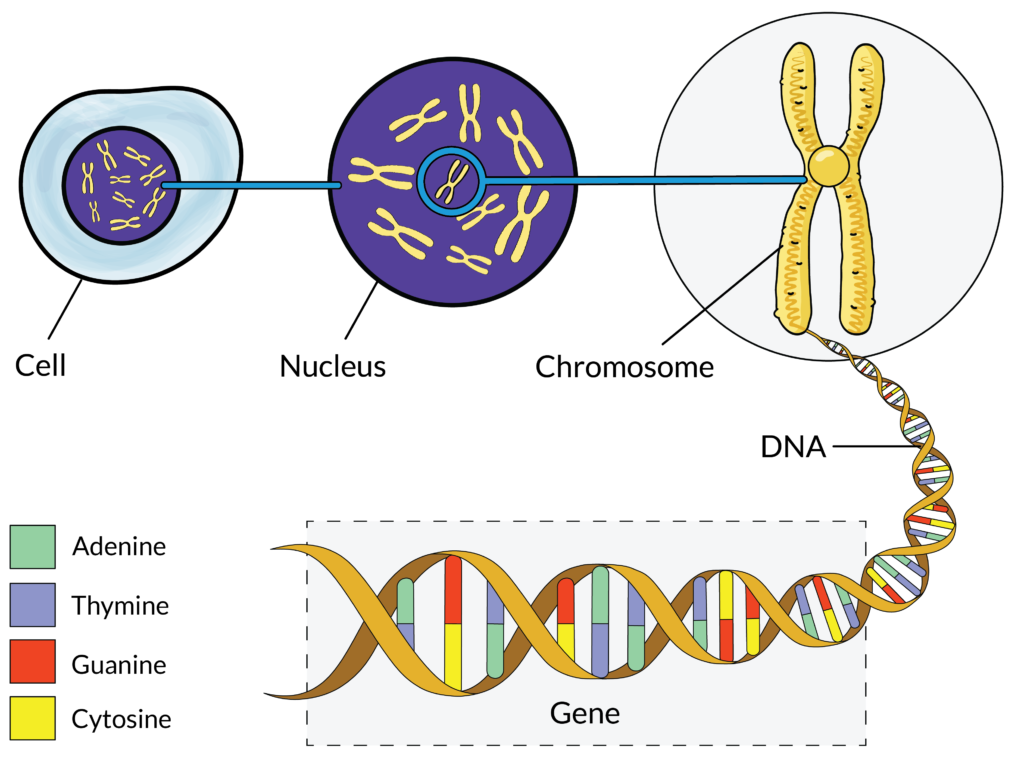
Genetics is the study, at a molecular level, of how our genes are passed down from one generation to the next. A sub-branch of genetics is cytogenetics. Cytogenetics is the study of numerical and structural variations in chromosomes. Myeloma is a genetically complex blood cancer and cytogenetics play an important role in the risk stratification of disease, since the prognosis of myeloma is mainly dependent upon chromosomal changes.
Cytogenetic testing is a type of genetic test performed in a laboratory and that looks at strands of DNA (deoxyribonucleic acid) called chromosomes that are housed deep inside cells. DNA is the genetic information that cells in our bodies need to develop, function and reproduce. Because of this, DNA is often referred to as “the instruction book for life.”
The information in DNA is written as a genetic code made up of four chemical bases: adenine (A), guanine (G), cytosine (C) and thymine (T). These chemical bases are organised into genes, which are small sections of DNA that our cells use to make proteins. Each gene is read similarly to how we would read a sentence in a book. Like letters in a word, the order in which the chemical bases of a gene are read determines which protein is made. Proteins have many functions in our bodies. For example, proteins provide structural support, strengthen our immune system (through antibody proteins), send messages (hormones), provide energy for our bodies and transport nutrients into and out of cells.
Like grammatical mistakes made when writing a sentence, errors can occur in our genes as well. For example, the chemical base “A” may be switched with a “T”, resulting in the production of a completely different protein. Many of these errors (also called mutations) are harmless. Other errors, however, can change the way a cell functions, lead to disease or disorders in our body and even cause cancer. There are many different types of gene mutations, which are discussed below.
Cytogenetic testing focuses on changes that can occur in chromosomes (e.g. broken, missing, re-arranged or extra chromosomes) and aims to identify the specific mutations found to cause disease and cancer. Identification of these mutations in cancerous myeloma cells can guide treatment decisions and provide more details on prognosis or the anticipated disease course.
How is cytogenetic testing performed in myeloma?
Cytogenetic tests are performed in a laboratory on a sample of bone marrow – the flexible and spongy inner layer of our bones where most of our blood cells are made. Bone marrow samples are usually taken by needle from the hip bone, under a local anaesthetic, in a procedure known as a bone marrow biopsy. While you are lying down on your side or your stomach, a sample of bone marrow is drawn up using a syringe. Bone marrow biopsies are often short procedures, and you may feel a sting, pain, or pressure as the test is performed. Most patients can go home right after the procedure. You may be advised to avoid strenuous exercise and heavy lifting for 24 hours after the procedure, and your medical team will tell you about any symptoms that you should seek care for such as pain, fever, swelling, or bleeding. Complications of bone marrow biopsies are rare and most people can resume their usual activities within 24 hours.
After collection, the bone marrow sample is then sent to a lab and later examined under a microscope by a specialist trained to evaluate cells and tissues. With specific lab tests, the specialist will then look for gene mutations that are commonly present in myeloma cells and report this information back to your oncology team. This cytogenetic testing process can take several days, or even weeks, to complete before you get results.
Why is cytogenetic testing helpful for myeloma patients?
Cytogenetic testing can help you and your medical team better understand the specific characteristics of your myeloma. Results may be used to determine your individual treatment options, as myeloma cells with certain mutations are expected to respond better – or worse – to specific treatments. In addition, cytogenetic testing can help determine the aggressiveness and risk of progression for your disease. Based on cytogenetics and other features, myeloma can be classified as either standard-risk or high-risk, the latter being associated with a less favourable prognosis. For more information on risk categories and treatment implications, please see the section on “What are the impacts of common genetic mutations in myeloma?”.
Like all genetic tests, it is important to discuss with your medical team whether cytogenetic testing is right for you currently. While some patients may appreciate the extra knowledge and insight provided by testing, others may find it difficult, psychologically, to be assigned a “risk category”. In addition, it is possible that cytogenetic test results can impact your eligibility – or ineligibility – for clinical trials as certain mutations may be listed in the trial exclusion/inclusion criteria.
What types of cytogenetic testing are available for myeloma patients?
There are multiple ways to test for cytogenetic abnormalities or mutations, including:
- Fluorescence In Situ Hybridisation (FISH) testing
- Karyotyping
These tests differ from one another in technique and reliability, and both are described in greater detail below. Notably, the availability, reimbursement and use of each of these tests in the clinical setting varies, and it is important to consult your medical team when determining which of these are appropriate for your individual medical care. Other techniques to evaluate genetic abnormalities exist and are described in the section titled “What additional genetic testing options exist for myeloma?”
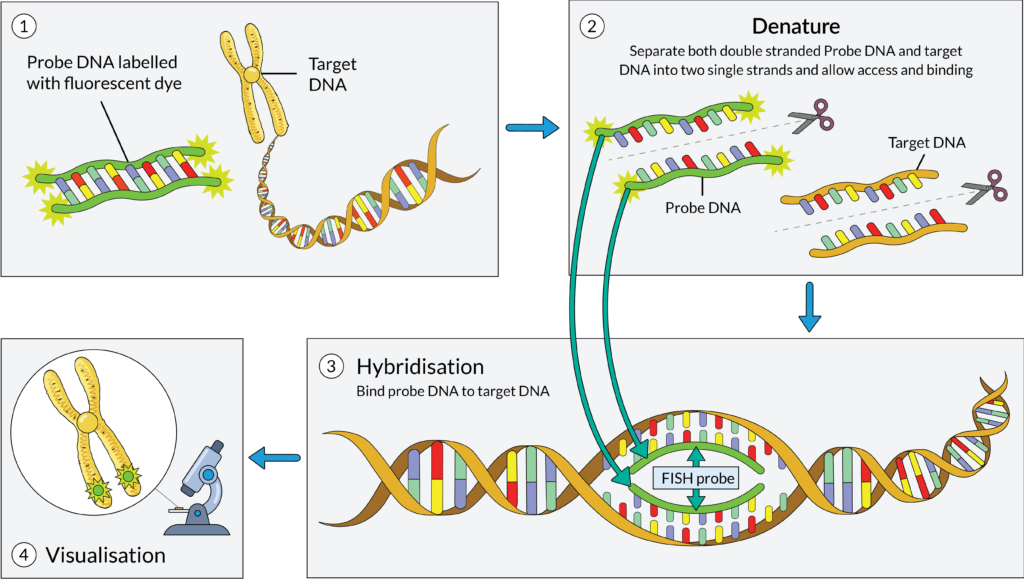
Fluorescence In Situ Hybridisation (FISH) testing
FISH testing is a type of cytogenetic test that uses fluorescent tags to visualise specific gene mutations that may be present in your cells. After your bone marrow sample is received by a lab, a technician prepares the DNA for testing. The technician will then create and mix the sample with DNA probes, which are short sequences of DNA with fluorescent dye that correspond to mutations commonly seen in myeloma cells. If the DNA probe attaches to the myeloma cell, it means the specific mutation is present in the cell. Results from FISH testing take time to process and vary depending on the particular lab and country, however, most results are available within 1-3 weeks.
FISH testing is the most frequently used cytogenetic test for myeloma patients, and it is generally regarded as the standard of care. However, availability of FISH may differ by country. Research has shown that FISH can detect more than 90% of known cytogenetic abnormalities in 60-90% of myeloma patients. The sensitivity and specificity of the test is reported to be greater than 95%.
Karyotyping
Karyotyping is an older method of cytogenetic testing that is still used today, albeit less frequently. Karyotyping allows technicians to see the size, number and shape of the chromosomes in a provided sample of myeloma cells. Unfortunately, research has shown that conventional karyotyping can only detect 20-30% cells with abnormal karyotypes, meaning many abnormalities can be missed. Although karyotyping is not as precise and efficient as FISH testing, karyotyping may still be useful when FISH is not available.
When and how often should I undergo cytogenetic testing?
To assess risk categories and determine treatment options, cytogenetic testing (when available) is performed most often at the time of diagnosis. In addition, some patients may undergo testing during first relapse to determine if new mutations have occurred that may be causing treatment resistance. Cytogenetic testing may also be performed multiple times throughout the course of the disease, since myeloma cells can change over time and acquire additional or different mutations to those that were present at diagnosis. These changes occur through a process called clonal evolution.
The timing and frequency of testing will depend on several factors including your individual disease, treatment exposure and response, whilst test accessibility and reimbursement will depend on the national policy. You should speak with your doctor if you have questions about undergoing testing.
What types of chromosomal changes can occur in myeloma cells?
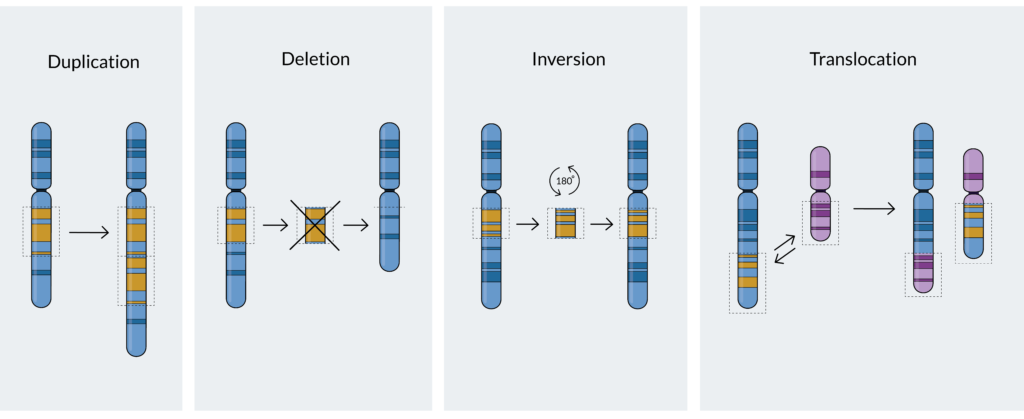
Various mutations can occur in myeloma cells and can affect either specific genes or entire chromosomes. The main chromosomal changes – or mutations – that occur in myeloma can be divided into the following categories:
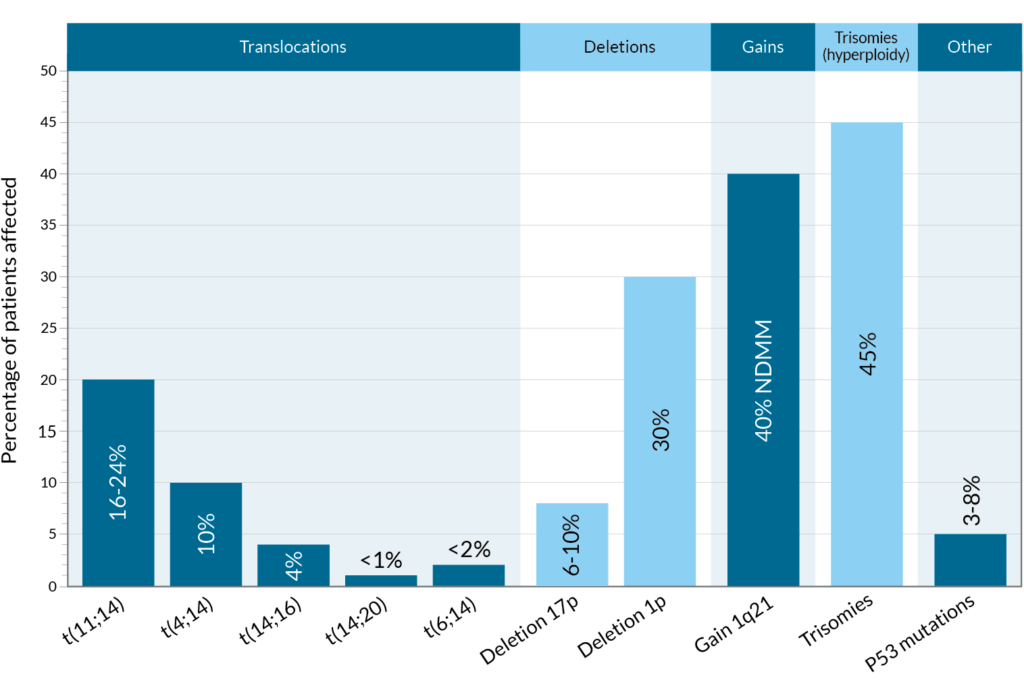
- Translocations: A translocation occurs when a part of one chromosome breaks off and reattaches to another chromosome, or when two chromosomes swap segments8. Translocations are named in the following way: t(A;B), where t stands for translocation and A and B represent the specific chromosomes that are Thus, t(11;14), for example, refers to a translocation (swap) between a part of chromosome 11 and a part of chromosome 14, which are two of the 23 pairs of chromosomes in humans.
- Deletions: A deletion occurs when one or more of the chemical bases in a gene sequence is erased. If you think of a gene sequence as a word, you can see how deletions can have an impact – what was originally TAG might now only read TG. Sometimes, only a single chemical base is deleted, and other times an entire gene can be deleted. An example of a deletion that occurs in myeloma is deletion17(p) – abbreviated del(17p), where p stands for a part of chromosome.
- Gains or amplifications: The presence of extra copies of genes, or parts of a chromosome, in a cell. An example of a common gain mutation found in myeloma is gain 1q21 (also written as 1q21+). In this case, a small amount of genetic material on chromosome 1 is abnormally duplicated on the long (q) arm of the chromosome at a location designated q21. Chromosomal changes with three copies are defined as gain(1q) while at least four copies are defined as amplification (amp)1q.
What are the impacts of common genetic mutations in myeloma?
Although various chromosomal abnormalities occur in myeloma, some mutations are more common and well-researched than others. In addition, some mutations are known to respond better to certain medications, whereas other mutations may result in treatment resistance and therefore aggressive disease.
For a full list, please see the full Q&A and appendices.
How are cytogenetics used to define risk categories and stages of disease?
How far or advanced cancer has become is often reported as the cancer “stage”. However, the approach to staging in myeloma is different from the typical staging systems used for solid tumour cancers. Myeloma staging uses cytogenetics and other characteristics to determine how advanced the cancer has become. The most commonly used staging systems for myeloma are the Revised International Staging System (R-ISS), which stratifies patients from stage I-III, and the mSMART model, which separate patients into two groups – standard and high-risk.
Although staging and risk stratification systems based on cytogenetics can be helpful when making treatment decisions and assessing the aggressiveness of disease, there are limitations to these systems, as well. For example, existing risk stratification systems do not account for the many other factors that contribute to one’s risk of progression such as age, the presence of other health conditions, frailty and overall health status. In addition, some patients may not meet the mSMART or R-ISS criteria for high-risk myeloma, however, experience poor disease response to induction or initial therapy. This then results in a patient being categorised as having functionally high-risk myeloma (myeloma that relapses within 18 months of treatment initiation and/or within 12 months of frontline autologous stem cell transplantation).
What additional genetic testing options exist for myeloma?
In addition to the cytogenetics testing described above, genomic testing and gene expression profiling (GEP) are two other types of tests that can be useful in assessing treatment options and aggressiveness of myeloma. Genomic testing focuses on the entire genome or genetic code of the myeloma cells and includes techniques like whole genome sequencing (WGS) or next generation sequencing (NGS) with the Myeloma Genome Project Panel. Gene expression profiling, by comparison, looks at the activity (expression) of genes within myeloma cells.
High-risk myeloma and cytogenetics
Cytogenetic testing plays a key role in identifying high-risk multiple myeloma, a subtype of the disease that tends to progress more aggressively and respond less favourably to standard treatments. Recognising high-risk features early enables haematologists to tailor treatment strategies accordingly.
Dr. Martin Kaiser of the Royal Marsden Hospital in London explains how high-risk myeloma is defined using cytogenetic abnormalities, particularly through FISH testing on purified plasma cells.
Summary
In summary, cytogenetics and other genetic testing options are useful tools to assess the characteristics of your myeloma, including treatment options, expected outcomes and risks of progression. Although FISH and karyotyping are currently the most common tests, they have limitations. FISH is the current “gold-standard”, but its sensitivity is limited. Genetic testing is an evolving field. Flow cytometry and next generation sequencing (NGS) are more sensitive methods, which are increasingly being used to detect mutations or measure residual plasma cells. They may be used for disease monitoring, or analysis of tumour plasma cells circulating in the blood, outside of the bone marrow. Those techniques are well known and used in research, but their use in clinical practice is still limited due to high costs and lack of expertise. It is likely that in the future, access to these tests will improve and new tests will emerge, changing the myeloma diagnostic and monitoring landscapes, and thus its treatment. The more we understand about the myeloma genome, the better we can treat the disease.
References
For a full list of references used to inform this patient information, please email info@mpeurope.org.