The MPE Advocate Development Programme (ADP) is a training programme directed to MPE members with the aim to provide them with the skills and knowledge that any myeloma patient advocate should have regarding the drug development process. The main goal of MPE is to put medicines research into a myeloma context and create a tailored training programme for the myeloma community.
Although there are other training programmes focused on medicines research, this is the first disease specific training programme focused on drug development and adapted to the needs of myeloma advocates, not only through theoretical content but also through practical sessions that will take place during the most important scientific meetings in Europe: the European Haematology Association (EHA) Annual Meeting and the European Society of Medical Oncology (ESMO) Annual Meeting.
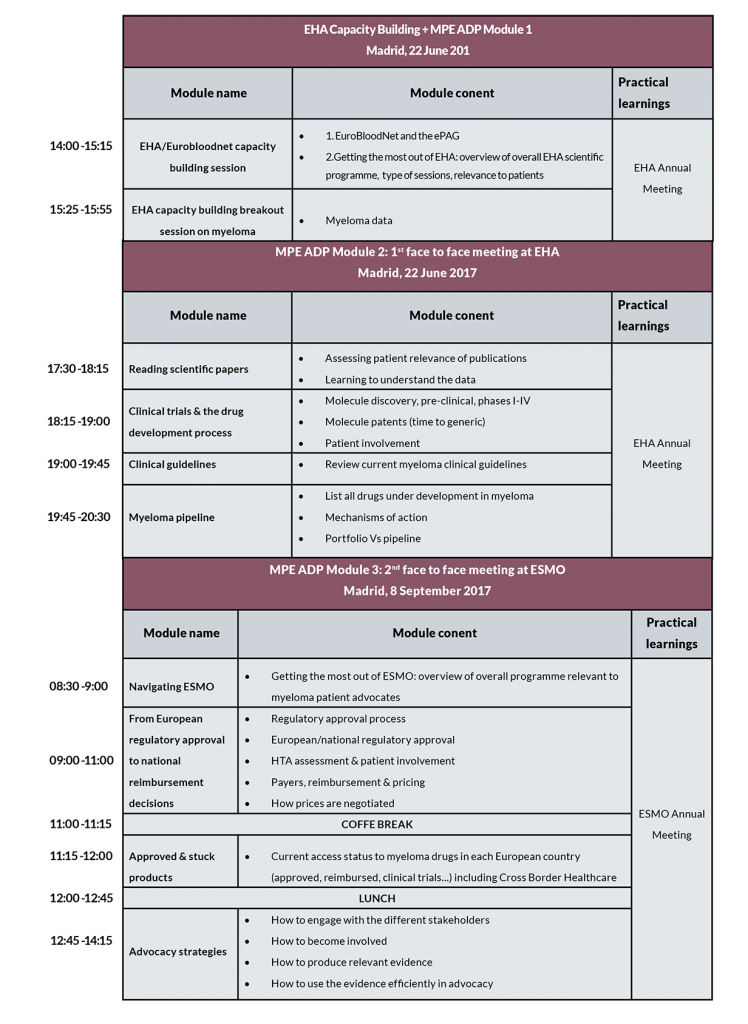
The MPE Advocate Development Programme will run from May 2017 to December 2017. This course will be taken entirely online with the exception of the two face-to-face sessions taking place during EHA (June 22-25, Madrid, Spain) and ESMO (September 8-11, Madrid, Spain). MPE will offer 5 scholarships to representatives of MPE member organisations to become part of this programme.
Ananda Plate, CEO of Myeloma Patients Europe, explains in this video the main objectives of this programme:
Applicants must meet all the following requirements:
- Representing an MPE member organisation
- Commitment to develop professional advocacy skills in the field of myeloma
- Commitment to invest at least 8 days of face-to-face meetings plus 20 independent learning hours (the scholarships include travel and accommodation to attend EHA and ESMO)
- Commitment to attend the two face to face meetings in Madrid (22-25 June and 8-11 September). The first face to face meeting will be held in connection to the EHA Capacity Building Meeting, which is also compulsory for any ADP trainee so it will be required to attend this programme to be part of the MPE ADP. EHA will provide 50 Fellowships for their EHA congress that include free access to the full congress and the EHA capacity building programme. The deadline to apply is 28 April. Please register as soon as possible via this link: https://www.surveymonkey.de/r/ZY5QQWJ
Content of the course
The MPE Advocate Development Programme consist of 3 modules, some of which will be taught in connection with the EHA capacity building programme: